Team develops solvent- and hydrogen-free method to upcycle high-density polyethylene plastics
20/08/2023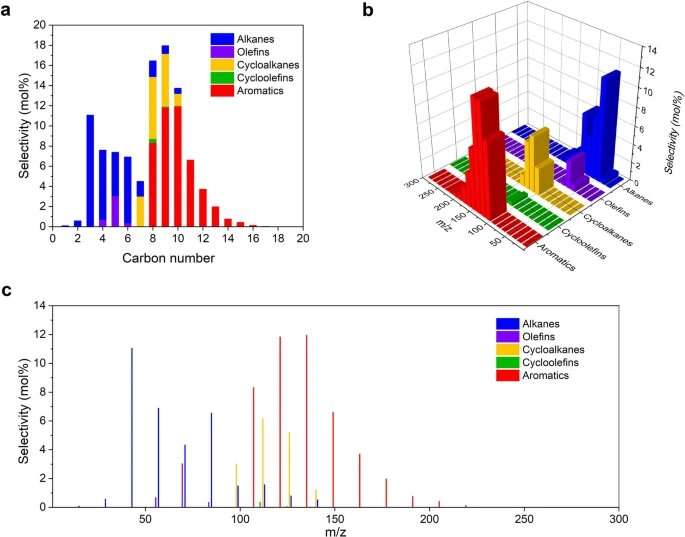
Detailed product distribution over Ru/HZSM-5(300) in LDPE upcycling at 280 °C for 24 h. Credit: Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01429-9
A research team led by Prof. Zeng Jie from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), has made a significant breakthrough in the field of plastic upcycling.
Their study, titled "Solvent- and Hydrogen-Free Catalytic Conversion of High-Density Polyethylene Plastics," introduces a novel dehydroaromatization and hydrogenolysis tandem strategy for converting high-density polyethylene (HDPE) plastics into valuable cyclic hydrocarbons without the need for solvents or hydrogen. The findings were published in Nature Nanotechnology.
Polyethylene, one of the most commonly used plastics, poses challenges in terms of natural degradation due to its stable chemical structure. Recycling technologies for waste polyethylene plastics not only mitigate pollution but also offer economic benefits.
Drawing inspiration from two processes in the petroleum industry, namely catalytic reforming of short-chain gasoline fractions and hydrocracking of heavy oils, the research team sought to treat waste HDPE plastics as a solid-petroleum raw material through environmentally friendly catalytic conversion, thereby producing downstream petroleum-based chemical products.
Inspired by two processes in the petroleum industry, the research team focused on catalytic reforming of short-chain gasoline fractions to obtain higher-value cyclic hydrocarbons, which generates hydrogen, and the hydrocracking of heavy oils to produce short-chain hydrocarbons, which consumes hydrogen.
Building upon these processes, the research team devised a "hydrogen-breathing" strategy for degrading high-density polyethylene (HDPE) plastics. They developed a molecular sieve-loaded metallic ruthenium catalyst (Ru/HZSM-5) that facilitates the dehydrogenation of the plastic into cyclic hydrocarbons, "breathe out" hydrogen in the process. Simultaneously, the plastic "breathe in" the released hydrogen and undergoes cracking, transforming into short-chain hydrocarbons.
The research team then explored the upcycling reaction pathways of high-density polyethylene plastics. They conducted catalytic experiments on the recycling of HDPE plastics with different molecular sieve loadings of ruthenium metal, and examined the effect of the molecular sieve pores on the reaction.
The results show that the HZSM-5 molecular sieve has a moderate pore size, which not only avoids the formation of thick cyclic aromatic hydrocarbons and carbon deposits, but also ensures the smooth desorption of cyclic hydrocarbons, thus guaranteeing the continuity and stability of the catalytic reaction. Ru/HZSM-5 catalysts have a very good cyclic stability, and are also suitable for different types of polyethylene plastics.
This research represents a significant advancement in plastic upcycling and holds great promise for the sustainable development of our society. By providing an innovative solution for the conversion of HDPE plastics into valuable cyclic hydrocarbons, this study contributes to the ongoing efforts to address plastic waste and promote a more sustainable future.
Source: https://tinyurl.com/yr5drjb7 via Phys.Org

