The Sun rises on perovskites
15/06/2023 Since perovskite solar cells were first reported in 2009, their record efficiency has gone from 3.8% to over 25%. Scarcely a week passes without a breakthrough unveiled in stability, efficiency or applicability. The world is awash with perovskite start-ups and spin-outs from top universities. And yet if you try to buy solar cells for a rooftop or campervan, you will be offered only silicon or perhaps less efficient thin film alternatives. Now the commercial launch of perovskite solar cells is planned this year, and the question of whether they will bear the burden of expectation becomes ever more urgent.A solar cell turns light into electricity. At its centre lies a layer of semiconductor whose energy levels are filled from the core up to a specific point (the valence band). Above this lies a region containing no energy levels (the bandgap) and then, at higher energies, a continuum of states that form the conduction band. In the material’s ground state, the conduction band is empty, but a suitably energetic photon striking the semiconductor may be absorbed, exciting an electron in the valence band to jump to the conduction band and leaving behind a positively charged ‘hole’ in the valence band. Separating the charge creates a potential difference across the semiconductor.
Silicon is, in several ways, not the ideal photovoltaic material. First, it doesn’t absorb light very well because it has an indirect bandgap – the highest energy valence electrons differ from the lowest energy conduction electrons in momentum. An electron therefore cannot pass directly between the bands by absorbing or emitting a photon – it must simultaneously exchange a quantum of vibration called a phonon with the crystal lattice. Such a three-body interaction is much less likely, so a photon travels much further through silicon before being absorbed. Silicon solar cells therefore cannot simply be printed in films but instead must be cut as individual wafers.
This increases the cost, and also exacerbates another problem: extracting charge from silicon is difficult. ‘If you have a dangling bond on the edge of silicon – so you come to the edge of the crystal and you’re missing further silicon atoms – you end up with a defect state right in the middle of the bandgap,’ explains physicist Henry Snaith of the University of Oxford, UK, one of the pioneers of perovskite photovoltaics and the co-founder of the spinout Oxford PV. ‘Electrons get localised in these defect states and can’t contribute to your solar cell performance.’ Premium, high-efficiency solar cells therefore need monocrystalline silicon.
Several direct-bandgap, thin-film technologies such as cadmium telluride and copper indium gallium selenide have never matched the efficiency of silicon and, as the cost of silicon falls, seem less and less attractive. Moreover, their expansion to the scale required to supply the world’s electricity demands would strain limited resources. In 2022, as total global installed solar power capacity ticked past 1TW, we can make the cells necessary to produce around 200GW power every year. To produce 100% of our energy from photovoltaics by 2050, this needs to increase to around 10TW. Snaith calculates that doing this using silicon cells would require on the order of three years’ worth of silicon production. Doing so using copper indium gallium selenide cells would require 400 years worth of indium, doing so using gallium arsenide cells 500 years’ worth of gallium and cadmium telluride cells 1000 years’ worth of tellurium.
Perovskite promise
Perovskites, however, attract the photovoltaics community for many reasons. Perhaps most importantly, they are easily made by crystallisation from solution. This doesn’t produce defect-free films, but it doesn’t need to. ‘These materials are almost entirely ionic,’ says Snaith. ‘When you have a defect, the energy of that defect is close to or in the continuum of states. The consequence is that we can have a very high density of crystal defects but they work very well for a solar cell.’ Moreover, the perovskites of interest are often made entirely from Earth-abundant materials, with 10TW installation requiring just a few days of lead production, for example.
Source: © Science Photo Library/Alamy Stock Photo
The perovskite structure allows a wide variety of cations and anions, both inorganic and organic
Perovskites are also extremely attractive for ‘tandem’ solar cells, comprising sequential layers with different bandgaps to make more efficient use of broad-spectrum light. Crystalline silicon will always have the same bandgap of 1.12eV – which lies in the infrared. Lower energy photons are not absorbed, whereas the excess energy from higher-energy photons is dumped into the lattice as heat. The perovskite structure, however, can encompass any material with the formula ABX3, where A and B are cations and X is an anion (or mix of anions). This presents chemists with an extraordinarily wide choice of possible compositions – and, crucially, the choice alters the bandgap.
Initially, the best results were found with methylammonium lead iodide (CH3NH3PbI3), which has a bandgap just over 1.6eV, but any of these ions can be partially or totally switched out. ‘We can swap the iodide at the X site for bromide or go all the way through to chloride, and you get a shrinking of the orbitals and a widening of the bandgap,’ explains Snaith. ‘So you go from a material that might absorb in the near infrared right through the visible to the blue and even the ultraviolet. We can also substitute on the metal site in group 14: going from germanium through to lead through to tin you can progressively reduce the bandgap.’
Perovskite problems
The main obstacles to perovskites’ commercialisation, however, have been instability issues. ‘If you take a silicon wafer and put it out in the rain, it’s going to be fine: it’s still going to be a silicon wafer,’ says electrical engineer Quinn Burlingame of Princeton University in New Jersey, US. ‘With a perovskite, you really, really have to work hard to avoid all the degradation modes that these things can undergo.’ Some of these are due to extrinsic factors, and can, in principle, be avoided through cell design. ‘These are salts after all,’ says Lynn Loo, who heads the Princeton group. ‘They are very susceptible to humidity, so the packaging needs to be right, the interfaces need to be right… it’s a whole slew of things that need to go right for the stability to be high.’ Others arise from intrinsic factors, and must be addressed by tailoring the perovskite itself. ‘You can’t avoid light – and a little bit of heating – from the Sun,’ says Burlingame.
Source: © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
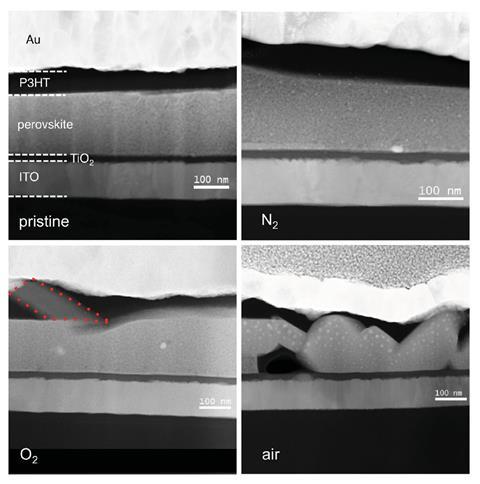
Methylammonium lead iodide will decompose over time in certain conditions
Many research groups have tailored the ionic compositions of their perovskites in highly variable, often opposing ways to improve stability. One of the main issues with organic–inorganic perovskites is the thermal stability of the organic ligand, especially methylammonium: CH3NH3PbI3 begins to decompose at 85°C, even in an inert atmosphere.
The field has therefore moved away from methylammonium, mainly towards the larger, less volatile formamidinium ion [CH(NH2)2+] – this also narrows the bandgap, making single junction cells more efficient. However, perovskites exhibit polymorphism, and they are only photo-active in the black cubic phase. Formamadinium is too large for the black phase of its lead iodide to be energetically favoured below 150°C. Instead, it forms the yellow hexagonal phase.

Source: © 2015 Macmillan Publishers Limited
In a variety of compositions, not all end up in the correct (black) phase
In 2015, researchers at the Korea Research Institute of Chemical Technology in Daejeon showed that adding around 15% methylammonium could stabilise formamidinium lead iodide’s black phase at room temperature. Subsequently, in 2020, Michael Grätzel of EPFL in Lausanne, Switzerland, and colleagues stabilised formamidinium lead iodide indefinitely by depositing it with methylammonium thiocyanate vapour.
‘If I had to buy a panel to put on my roof…there is no doubt the main phase would be formamidinium,’ says Grätzel, Snaith’s former mentor at EPFL, whose group subsequently arrived at many landmark results independently ‘We have a new stabilised efficiency record [in a single-junction cell] we published [in January] in Science with the group of Li Xiong from Wuhan…That was a formamidinium-based perovskite.’
Working in tandem
Loo’s Princeton group, meanwhile, is studying the benefits of abandoning organic ligands altogether and using a simple metal A site cation. Unfortunately caesium – the largest viable option – is too small for the black phase to be stable at room temperature. Instead, below 300°C CsPbI3 also turns yellow as it enters the orthorhombic phase. Loo’s group shrinks the grain size, which increases the surface area relative to the bulk and thereby disfavours the undesired phase change.
CsPbI3 does have a slightly wider bandgap (around 1.73eV), which reduces the efficiency of single junction perovskite cells, but Burlingame believes that this is a price worth paying. ‘We took the more stable options across the board because that gives us first a proof of concept that a properly optimised perovskite solar cell can be stable and secondly a baseline that allows us to engineer towards greater efficiency, and I think that’s kind of opposite to the way this field has always gone,’ he says. In any case, he adds, ‘In recent years the cost of silicon has dropped so sharply that people aren’t as much looking for a replacement, they’re more looking for a way to complement and supplement silicon.’
Source: Based on © Shutterstock
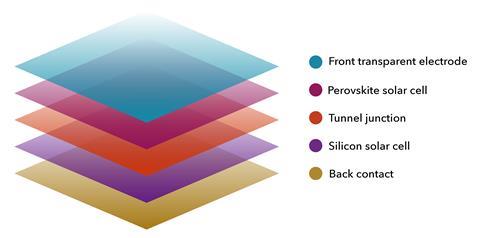
Perovskite materials are most likely to be used as just one layer in a tandem solar cell, to harvest wavelengths not used by silicon
Oxford PV agrees with this rationale and is this year planning what may be the first commercial sale of tandem solar cells with perovskite on silicon, designed to be integrated in conventional solar panels. Adding this perovskite top layer could increase the efficiency of commercial cells from around 24% to 27%, the company claims. Oxford PV does not disclose its solar cell’s composition. Referring to his research work, however, Snaith says that ‘ironically, the organic–inorganic perovskite is actually more stable. This probably is due to the hygroscopic nature of caesium halide salts… Over 25 years in the field there will be some humidity that gets into even the very best encapsulated cells.’
Describing the attraction of perovskite on silicon tandems, Snaith says that ‘if you can increase the efficiency of a module by say 20%, and you haven’t driven up the cost of producing the module by very much, you more or less get that 20% reduction in the cost of the electricity you generate… We’re literally piggybacking on the existing industry.’ He acknowledges, however, that, if perovskite solar cells are as successful as he and other researchers hope, the eventual outcome should be multi-layer tandem perovskite solar cells.
Kai Zhu and colleagues at the US National Renewable Energy Laboratory in Colorado, as well as several other academic laboratories and commercial startups, are researching multi-layer tandem cells (although Zhu notes the NREL researchers are developing useful perovskites for any and all applications). They – like some other groups – produce methylammonium-free perovskites by mixing caesium and formamidinium at the A site. ‘[Formamidinium] is pappa bear – too fat; caesium is baby bear – too thin; you put them together, you can get into the Goldilocks zone,’ explains NREL’s Joseph Berry.
Producing an ideal tandem solar cell requires tuning the bandgap of the top and bottom cells to complement each other. For the wide-bandgap top cell, this often involves mixing iodide and bromide at the X site. This can lead to phase segregation – when the two salts either crystallise separately rather than forming a mixed film or migrate through the film over time. Zhu and colleagues have shown that this can be dramatically reduced in their own cell by changing the method of drying the film. Lowering the bandgap of the bottom cell often involves mixing lead and tin on the B site, which is challenging because tin perovskites or mixed tin–lead perovskites are usually much less stable than pure lead perovskites, partly because the Sn2+ ion can easily be oxidised to Sn4+, causing the decomposition of the perovskite lattice. The NREL researchers have unveiled a solar cell that mitigates this issue too, and moreover the top cell in a tandem junction provides the bottom cell with some protection from high energy photons most likely to cause oxidation.
Strong and stable
With multiple groups trying to push various perovskite solar cell designs from the laboratory to the market, it becomes increasingly important to develop standardised methods to compare their long-term durability. Extrapolating how long a perovskite solar cell will last, however, is tricky. There is no perovskite-specific ‘accelerated ageing’ test because no-one truly knows how perovskite solar cells will degrade after – hopefully – 25 years or more in the field. ‘The test that people throw around a lot is sometimes called an 80–80 test or damp–heat test, which is thermal cycling under 80% humidity from –60°C up to 80°C a ton of times, and essentially that’s supposed to produce stress on the encapsulation and delamination of layers in a silicon module, which is how silicon fails these days,’ explains Burlingame. ‘They’re not testing the silicon turning into a different polymorph or some chemical species coming in and attacking the silicon… It’s like the serpent that eats its tail: you kind of need to know the degradation modes that matter to choose the test, but you also need to run some tests to see which degradation modes matter.’
Loo’s group estimated the chemical stability of the perovskite itself at rooftop temperature by testing it at a series of higher temperatures and plotting how the lifetime varied. ‘If the degradation mechanism is thermally activated and you can see that all your data collapses into one specific functional form, then you can say that it’s one specific mechanism or multiple mechanisms with the same temperature dependence,’ says Loo. ’This is the first thing one should try if you have a very stable cell, and then you can extrapolate a lifetime if you want to. But you need to make sure the data fall in place before you move on to subsequent analysis.’ In 2020, multiple experts from across the world, including all those interviewed here, published a consensus statement proposing a set of procedures for testing and reporting the stability of perovskite cells based on known degradation modes of perovskites.
The published records for stability (as well as efficiency) in perovskite photovoltaics have all involved lead halides, so this is the focus of the vast majority of research and development. (The Chinese company Microquanta has reportedly promised to bring a tin halide semiconductor to market this year, but further details remain unclear.) This has led to concern about the safety and marketability of products containing soluble salts of a toxic metal.
Lead will probably preclude the use of perovskite-based photovoltaics in children’s toys that might be eaten
Grätzel is cautious: ‘We have to be extremely careful with lead,’ he says. ‘1mg ingested for a person of my size is already toxic. For large-scale, field solar farms with a fence around them, I see no problem. Rooftop may be OK – I have silicon solar cells on my roof and it wouldn’t occur to me to go up there and start fiddling with those. But when people start talking about flexible cells, I would say that’s a no-go: for consumer products we absolutely cannot tolerate lead.’
Snaith remains bullish, noting that a perovskite research project co-funded by the EU’s Horizon 2020 had conducted an environmental impact assessment on perovskite on silicon tandem cells and concluded that the environmental impact of adding the perovskite was positive. ‘We looked at the ecotoxicological impact of all the different materials, steps and layers,’ he says. ‘The silicon accounted for just over 40% of the environmental impact, and that was basically related to the power that went into producing the silicon wafers, the indium from the indium tin oxide accounted for another 30%, mainly related to the mining of the indium. The lead from the lead halide perovskite, assuming it was fully emitted into the environment – which would not happen – amounted to about 0.3% of the overall ecotoxicity, so you’re actually having a positive environmental impact by offsetting the ecotoxicity of the silicon.’ Berry remains equally skeptical of toxicity concerns. ‘Lead will probably preclude the use of perovskite-based photovoltaics in children’s toys that might be eaten, but even if your kid ate it, we think we could sort that,’ he says.
Asked to name the single biggest issue facing the stability of perovskite solar cells, Snaith laughs. ‘I’ll argue that there is no issue,’ he says; ‘There’s certainly things you need to get a handle on to make the stuff stable. It’s very easy to make unstable perovskite solar cells.’
Tim Wogan is a science writer based in Oregon, US
Source: https://l1nq.com/mR9dk via Chemistry World

