Microfabrication makes microdroplets 'pattern-obedient' but Gibbs equation 'disobedient'
18/04/2024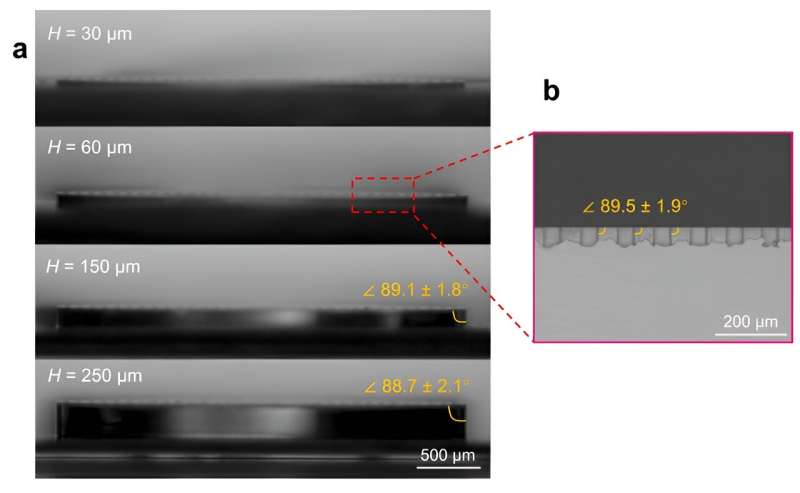
(a) Optical images showing the outmost edge angles of the circular closed-loop surfaces with varied height (H). (b) SEM image of the cross-section of the closed-loop structures for H = 60 μm. All images show that the edge angles are around 90° with negligible deviations. Credit: Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2315730121
Microdroplets find versatile applications in fields of chemistry, materials science and biochemistry, particularly in chemical engineering and biochemical microfluidics like microreactors and biosensors. Achieving precise control over microdroplets in their shape, size, and contact angle (CA) is especially crucial for the applications like precise control of the printing/coating patterns and chemical reactions.
Current research leverages the capillary and the edge effects of micropillared structured surfaces to achieve certain polygonal patterns of liquid droplets. However, when given a specific liquid/material combination, especially for superhydrophilic (or completely wetting) surfaces, the achievable contact angle is limited by the conventional Gibbs equation typically used to access the CA of a macro-droplet on rough surfaces. The contact shapes of the microdroplets are limited to certain polygons. Achieving precise control over microdroplets with arbitrary shapes and a wide range of CAs has long been a challenge.
In a study published in the Proceedings of the National Academy of Sciences, Prof. Gao Yurui's group from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences, collaborating with Prof. Zeng Xiaocheng from City University of Hong Kong and Prof. Francisco Joseph S. from the University of Pennsylvania, employing photolithography techniques and subsequent processing, fabricated a class of structured surfaces featuring concentric closed-loop microwalls/microchannels, which allows for precise control of microdroplets with wide range of CAs, and high shape and pattern tunability.
Based on the notion of "topological wetting states," the researchers engineered a variety of surfaces with homocentric orthorhombic closed-loop microwalls/microchannels using lithography techniques. These surfaces exhibited precise microwall edge angles of 90° and, with the application of UV/ozone treatment, achieved an intrinsic contact angle of 0°. On these surfaces with closed-loop structures, topological wetting states were observed.
Due to the closed-loop topology of the surface structures, the microdroplets exhibited multiple Wenzel states with their three-phase contact lines pinned at the outer edge of the microwall. and the CA can be varied widely from 0 to 130°. By designing the shape of homocentric microwalls, the microdroplet contact area and size can be effectively controlled as well, enabling formation of not only regular shapes such as circles, triangles, and squares, but also of irregular patterns like heart-shaped shapes.
In addition, the researchers extended the control to the dimension of CA. They proposed control across a broad range (from 0 to >130°), particularly for intrinsically completely wetting surface/liquid combinations, by leveraging droplet evaporation and the close-loop geometry.
Interestingly, the researchers uncovered a wetting phenomenon that challenges the traditional Gibbs equation in describing droplet at its boundaries: regardless of the shape of the closed-loop structure, the maximum CA of the microdroplet remains stable at around 130°, largely deviating from the angle limit predicted by the Gibbs equation based on macroscale edge effects.
This finding suggested that the Gibbs equation, traditionally used for accessing the CA of macrodroplets on rough surfaces, may not be applicable at the micro or nano scale. This conclusion is applicable to various liquids, including isopropanol, ethanol, decane, and octane considered in this study.
Through independent molecular dynamics simulations, the researchers attributed this large deviation from Gibbs equation prediction to a cumulative effect of water-surface interaction and the atomic structure of the edge. They suggested adding a correction term to the Gibbs equation to address the apparent deviation.
"This work demonstrated closed-loop microstructures with well-controlled orthorhombic edges, enabling a comparative analysis of the droplet's contact angle and the edge angle. It provides compiling evidence on the need for the modified Gibbs equation at the micro or nano scale and the obtained droplets that can be precisely controlled offer a possibility for accurate measurement of droplets.
"It has implications for exploiting controllable microdroplets in fields such as microfluidics, chemical reactions, and biosensing, offering new opportunities for material manufacturing and green synthesis," said Prof. Gao.
Source: https://tinyurl.com/y5jjbb8c via Phys. Org

